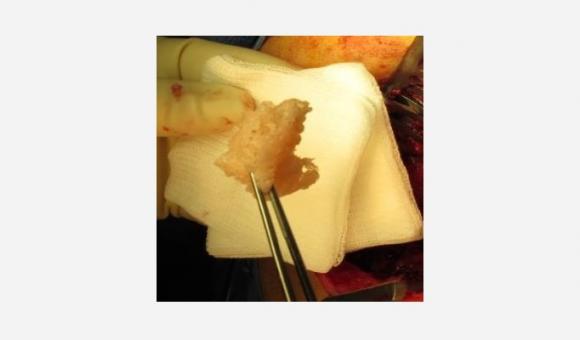
Novadip, a spin-off of the University of Leuven, has developed a revolutionary therapeutic product. Using stem cells selectively taken from the fat of a patient, these Walloon researchers have succeeded in forming a bone graft. Known as Creost, this piece of bone has the appearance of plasticine. It is designed to facilitate bone reconstruction in patients. With this discovery, Novadip intends to conquer international markets.
Created in 2013 on the basis of the research work of Professor Denis Dufrane, as part of the University of Leuven and the Cliniques Universitaires Saint-Luc in Brussels, Novadip is a promising young start-up in many ways. This company, active in the pharmaceutical sector, owes its birth to a technical and medical discovery. "From the fat of a patient, a sample smaller than a sugar cube punctured in the stomach, we can isolate adipose-derived stem cells. Thanks to a particular signal that is transmitted to them, these cells can be differentiated. Depending on the signal they receive, the cells can reconstruct various tissues including bone tissue having all the properties of native bone”, says Jean-François Pollet, CEO of Novadip.
Researchers from the University of Leuven have therefore succeeded in synthesising a bone from stem cells punctured in a patient. This is a world first and the fruit of research begun in 2007 by the teams of Prof. Dufrane, head of the Tissue and Cellular Therapy Centre of the Cliniques Saint-Luc. Novadip was set up to develop this concept of regenerative medicine and to transfer this innovation to the market. "At the heart of our laboratories, these stem cells taken from the patient are therefore placed in culture, and differentiate into osteoblasts, cells capable of synthesising a new healthy bone tissue capable of correcting any injury or degeneration of the skeletal system," continues the CEO. "The revolutionary nature of the approach lies in the fact that we manage to create, in three dimensions and without any limits in terms of graft volume, bone tissue with all the qualitative and mechanical properties necessary for its implantation in severe bone injuries and diseases, without adding any biomaterial."
Using a solid graft, in three dimensions, has an advantage over stem cells. The problem with these is that you can never fully control their immobilisation on a location, or the quality and consistency of the tissue to be regenerated. With Creost, Novadip has succeeded in finding a solution to this problem.
Therapeutic applications
Today, Novadip intends to develop the results of this research, to apply them through a medical procedure that can help many patients with severe bone injuries or degeneration. "This discovery should be able to help patients suffering from cancer of the bone, a non-consolidated fracture, congenital pseudarthrosis, intervertebral disc degeneration or maxillofacial trauma”, comments Jean-François Pollet.
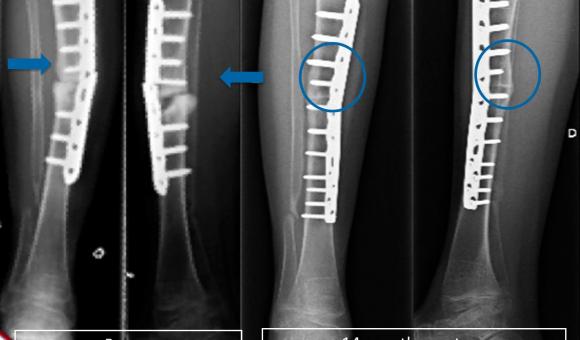
But before we get to that point and be able to market Creost, the start-up still has some way to go. From the results of the fundamental research phase, it was first necessary to develop the "proof of concept", i.e. translate the results of this research into a medical application. Before testing the concept in human patients, it went through the animal testing phase and delivered extremely convincing results. This stage has already been completed for some time. "At present, eleven patients with bone consolidation problems, or degeneration of the spine or a bone tumour have received the treatment we offer, with excellent results," says Jean-François Pollet. "Within a few months, we have witnessed a complete bone reconsolidation and some of these patients have been monitored for more than three years without any side effects or degeneration of the transplant being observed."
Marketing on the cards
The challenge now is to industrialise the process. Nodavip, incorporated with a startup capital of 550,000 euros, provided by its two founders, Jean-François Pollet and Denis Dufrane, Sopartec – the UCL’s technology transfer tool – the Vives II funds and St-Luc Clinics , is currently actively raising the 8.5 million euros of funds necessary. The start-up is in discussions with several investors that should enable it to put together the amount needed for the completion of a multi-centre clinical study at international scale. It will aim to demonstrate the safety of the treatment and its effectiveness. It should take around three years. "This is part and parcel of registering any new drug. There are two or three phases. After this first step, it will be necessary to conduct additional clinical studies, which will involve much broader panels of patients," says Jean-François Pollet.
The actual marketing of the treatment is expected around 2019-2020. In the meantime, Novadip will still have to carry out two more funding rounds but the start-up’s bosses are confident. The investors encountered so far are showing a keen interest in what they have to offer. However the challenge is immense, both in medical terms and from an economic point of view. "For marketing purposes, it will be necessary to develop two pharmaceutical entities, one for the European market and one for the US," says Jean-François Pollet. "They are currently the two main markets of this pharmaceutical sector, but that doesn’t mean that we are overlooking the other markets," points out the CEO.
Novadip, in collaboration with the Saint-Luc clinic, has other research and development projects. Today, the treatment is part of an autologous approach – a graft is created from the patient's own stem cells. But the start-up also hopes to enable allogeneic transplants, in other words supply bone grafts created from a donor’s stem cells, thus making it possible to treat several patients at the same time. "With this approach, however, it will be necessary to consider the problem of transplant rejection," says Jean-François Pollet.
If Novadip is successful in creating bones, it is also possible, from stem punctured in the fat of any individual, to create other types of tissue or organs. "Further developments in already advanced stages have generated convincing results. Provided that we know what signal to transmit to them, it is possible to create any tissue using stem cells. At this stage, however, we do not wish to communicate these results."